
Post-Transplant Complications Pose Risks
for Allograft Function
In the United
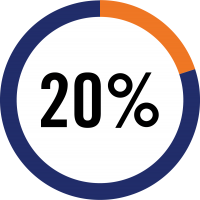
show subclinical
acute rejection
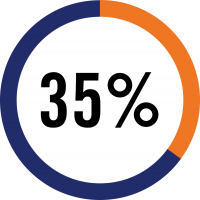
surveillance biopsy
in the first year
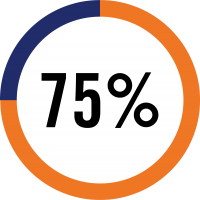

TruGraf® is the first and only non-invasive test covered by CMS as an alternative to surveillance biopsies to rule out silent subclinical acute rejection in transplant patients with stable graft function.
Despite improvement in short-term outcomes, long-term outcomes for kidney transplant recipients remain suboptimal. Immunological rejection is a leading cause of graft failure and recent research points to undetected “silent” subclinical acute rejection (subAR) in patients with stable renal function as a key component of this problem.
Traditionally the diagnosis of silent subclinical rejection has required a surveillance biopsy on a stable patient, but it’s widely acknowledged that a non-invasive method would offer significant advantages especially in terms of patient safety. TruGraf®, a clinically-validated, blood-based gene expression assay, is the first and only non-invasive test designed and validated to rule out silent rejection, offering the potential to limit use of surveillance biopsies to patients most likely to benefit from them.

With the outbreak of COVID-19, transplant programs are minimizing office visits, both to ensure patient health and safety, as well as reduce the burden on staff so they may focus their care on patients who are most in need. To help physicians continue to provide state-of-the-art care for their patients without requiring them to visit the transplant center, we offer a comprehensive catalog of tests – all of which can be run from samples collected remotely – via our mobile phlebotomy service, Labs@HOME.
The Labs@HOME service allows physicians to order and customize a limited menu of infectious disease and transplant-related testing. The specimen collection is performed safely and securely by a qualified phlebotomist who will work with the patient to schedule an appointment at the patient’s residence. This ensures routine transplant testing is available to high risk patients in their homes—eliminating the need to visit the hospital or transplant center when it is not critically necessary.
